20th International CODATA Conference (Times
New Roman, 11pt)
Session: Computational informatics: integrating data science with materials
modeling
Estimation of Solvent Effects For
Complexing Reaction of Propylene and Nickel Dithiolene
Qing-Zhen Han1, 2, Yue-Hong
Zhao1 and Hao Wen1
1Multi-Phase Reaction
Laboratory,
2Graduate University of Chinese Academy of Sciences,
P.O. Box 4588, Beijing 100049, P. R. China
The formation of olefin complexes
is of potential importance in the separation of olefins. The solvents will
affect the activation energies, and hence the rates and equilibrium constants
of the complexing reactions, which performance should
be well estimated for the purpose of industrial practice. The solvent effects
on the complexing reaction of propylene and nickel dithiolene Ni(S2C2H2)2
+ C2H4=CH2 ® Ni(S2C2H2)2×C2H4=CH2 are studied in
this work by using B3LYP method and Onsager model.
Complete optimizations of all the stagnation points are performed in benzene,
toluene, tetrahydrofuran, dichloromethane,
1,2-dichloroethane, acetone, ethanol, methanol,
1,2,3-propanetriol, dimethylsulfoxide and water, respectively. The reaction of complexing nickel dithiolene with
propylene is a two-step process:
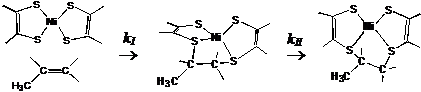
The activation energy of the
step-I is higher than that of step-II, indicating that the step-I is the
rate-determining step. The solvents make slight changes in the geometries of
the reactants, transition states, intermediates, and products. However, the
activation energies of step-I (or step-II) will exponentially decrease from 125.0 to 113.0 kJ×mol-1 (or from 101.8 to 83.43 kJ×mol-1) when the dielectric constants of
solvents increase from 1.00 to 78.39, while the reaction rates of step-I (or
step-II) will exponentially increase from 0.767´10-9 to 96.2´10-9 s-1 (or from 0.550 to 1.04 s-1), and the equilibrium constants
will rapidly increase from 0.1863 to 126.4 l×mol-1.
Also, the sharp
variations of activation energies, rate constants, and equilibrium constants
will appear when the value of
the dielectric constant is lied between 1.00 and 20.70, while these variations
will become mildly when the dielectric constant of solvents is larger than
20.70. All of these results
demonstrate that the complexing reaction of propylene
and nickel dithiolene will become much easier and
faster to occur in polar solvents. The relationship between the equilibrium
constants of the complexing reaction ![]() and the dielectric
constants of solvents e can be presented mathematically as
and the dielectric
constants of solvents e can be presented mathematically as ![]() with the correlation
parameters A = -139.3 l·mol-1, B = 129.1 l·mol-1 and t = 21.17. This relationship may be seen as a reference for solvent
selection in olefin separation practice.
with the correlation
parameters A = -139.3 l·mol-1, B = 129.1 l·mol-1 and t = 21.17. This relationship may be seen as a reference for solvent
selection in olefin separation practice.
Keywords: Density
functional theory; Solvent effects; Performance estimation; Olefins; Nickel dithiolene